First in HumanInnovationPartnershipTherapeutic Imperial-developed cancer drug enters phase I clinical trial
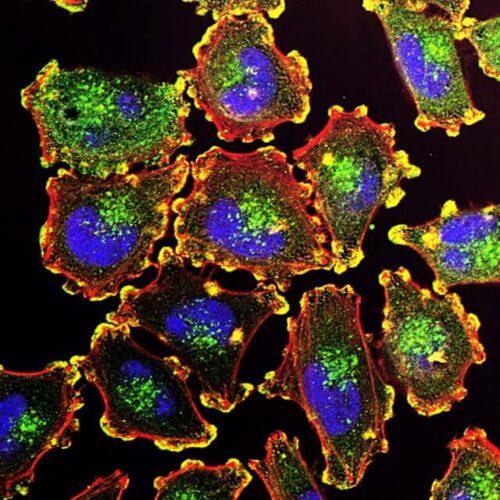
Appropriate functionality and propagation of normal cells is controlled by the action of specific enzymes that regulate cell growth, DNA replication and cell division. Cancer development and progression occurs through acquisition of mutations that subvert the action of enzymes that constrain cell proliferation. Two pathways critical for appropriate control of normal cell function and proliferation that are subverted in most cancer types, are the processes of cell cycle progression and regulation of gene expression. CDK7 acts as a master regulator, bridging the process of gene expression and as the enzyme that is needed for activation of kinases that direct cells through the cell cycle (growth, DNA replication, cell division).
Reasoning that inhibiting CDK7 would target cancer cells at multiple levels, a large collaborative team led by researchers in the Departments of Chemistry and Surgery & Cancer at Imperial, in collaboration with researchers at Emory University (Atlanta), and supported by EPSRC, Cancer Research UK and by the NIHR Imperial BRC, developed a highly selective, oral CDK7 inhibitor, which demonstrated potent anti-proliferative properties. This drug discovery was significantly enabled by computational drug design and took the project all the way from a clinical hypothesis and virtual chemical scaffolds to a derisked clinical drug candidate. Pre-clinical in vivo evaluation of the candidate drug by the Imperial group, funded by Cancer Research UK, showed its high promise as an effective novel treatment in several tumour types.
Additional pharmacological and in vivo studies, in partnership with Sygnature Discovery (Nottingham, UK) and Carrick Therapeutics (Dublin), have confirmed the initial promise of this first-in-class selective CDK7 inhibitor, ICEC0942, and have facilitated approval for phase I clinical trial. ICEC0942, referred to as CT7001, is currently being evaluated as a monotherapy in advanced solid malignancies in a phase I trial sponsored by Carrick Therapeutics, with three participating recruitment sites: Imperial College Healthcare NHS Trust in London, The Christie NHS Foundation Trust in Manchester, and the Churchill Hospital in Oxford. The first patient was dosed in December 2017, and the study continues to recruit patients.
The NIHR Imperial BRC continues to support the cross-College discovery team which aims to develop new compounds and, exploiting investment in NIHR Imperial BRC-funded research infrastructure, combine biological and clinical outcome data to influence therapeutic decision making.
Licensing of the technology was led by Cancer Research Technology with support by Emory University and Imperial Innovations, the Technology Transfer Office of the BRC.