Guideline Pioneering progress: clinical trials team showcases innovation and excellence
Imperial Clinical Trials Unit (ICTU), an NIHR Imperial BRC-supported facility, is at the forefront of the evolving clinical trials landscape, working to advance trial methodology and pioneering innovation and excellence in clinical trials. As part of this, the unit recently had 15 presentations selected for the 7th International Clinical Trials Methodology Conference (ICTMC) in Edinburgh.
ICTMC is an important event for researchers involved in clinical trials covering cutting-edge work in trial methodology, with delegates attending from all around the world to share ideas and approaches.
Staff presented both in person and via posters the latest findings from our work. Covering topics such as public perspectives in clinical trials, study design and methodological approaches, data management, technological innovations and statistical methods.
Here, two ICTU staff members, Kenan Direk, Deputy Head of Clinical Data Systems and Arzish Haqqee, Clinical Trials Manager share their experiences of presenting at their first clinical trials conference:
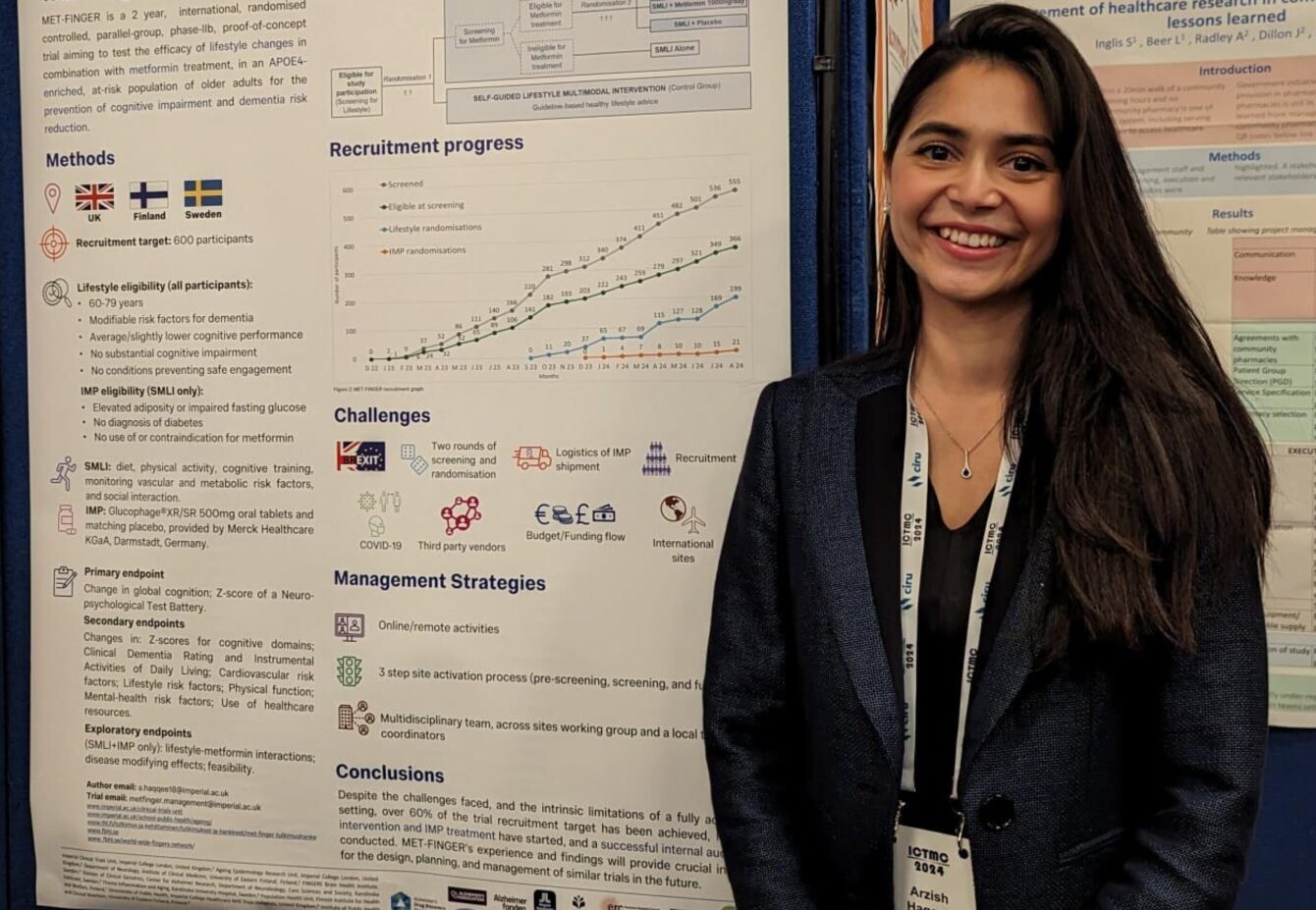
Arzish Haqqee
“I presented a poster on the progress and challenges of the MET-FINGER study. The first randomised controlled trial combines lifestyle changes and metformin for the prevention of cognitive impairment in an academic setting. Some of the challenges we faced included the impact of Brexit and COVID-19, logistics of investigational medicinal product (IMP) shipment, third-party vendors and working with international sites across the UK, Sweden and Finland. These were overcome by utilising online and remote activities and a multidisciplinary team working across sites with local coordinators. We’ve had a successful internal audit; all sites are now active and we have achieved over 60% of the trial recruitment target.
“It was great to attend such a well-organised conference with a comprehensive programme including a range of parallel sessions which ensured there was something of interest for everyone.
“There was a diverse representation of attendees, insightful Q&A discussions and opportunities for networking during the poster sessions.
“It felt exciting to represent ICTU and showcase some of the work in our growing portfolio of trials. Being a part of a supportive and collaborative environment is inspiring and motivates me to continue contributing towards impactful and meaningful research.
“The conference also highlighted the importance of inclusivity in clinical trials, efforts towards creating greener trials, and always ensuring that trials are designed with patients and the public in mind.”
Kenan Direk
“Increasingly sophisticated clinical designs are pushing traditional data management approaches beyond their operational limits. We know that teams often face bottlenecks because certain tasks have, for historical reasons, been performed in a specific way. These legacy procedures frequently fail to scale effectively and result in significant personnel-related costs.
‘I presented some of our group’s recent work that addresses this: around the replacement of manual processes with scalable, efficient alternatives using common, open-source software.
“Enacting this change is not a simple task. It requires a strategic operational rethinking. Combined with empowering our teams with the right tools and skills needed to adapt to evolving modern demands faced by clinical trial units.
“This was both my first clinical trials conference and my first representing ICTU. It provided a valuable opportunity to connect with others tackling similar challenges and learn about the strategies they’ve employed to address them.
“We were very fortunate that so many ICTU colleagues had their work selected for talks and posters, it gave a real sense that what we’re doing has a tangible impact within the trials community.
“I have a longstanding interest in the use of Trusted Research Environments and the use of routinely collected data, so to learn about others’ experiences resonated deeply, particularly with the practical and governance challenges to this type of work.
“Change is very much needed, and I am hopeful that we can play a role in shaping this area moving forward.”
Conference reflections
“We’re proud of our representation at the conference, it reflects our unit’s experience in trial design, delivery, and methodology.
“As one of the larger clinical trial teams present, it was great to contribute through our presentations as well as through active participation in discussions.
“We are eager to apply what we’ve learned to further enhance our practices. In particular, the learnings about decentralised trial methods and adaptive trial approaches are immediately relevant for our work, and we are looking forward to implementing them.”
