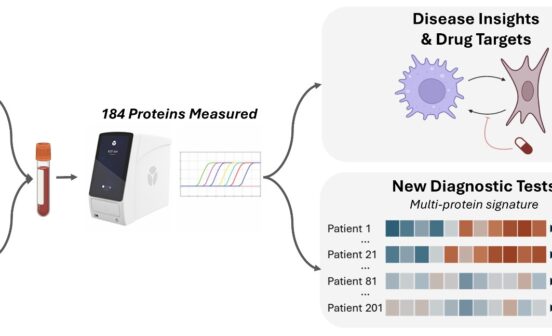
Therapeutic Renal disorders
Our research has led to a number of collaborations with pharmaceutical corporations such as Baxalta, Rigel Pharmaceuticals and Boehringer Ingelheim covering a number of renal disorders. We have also influenced public policy in the UK.
In glomerulonephritis – an inflammation of the kidneys – we have discovered novel therapeutic targets. Polymeric IgA from patients with IgA nephropathy (IgAN) stimulates human mesangial cells to proliferate and produce cytokines. These effects were blocked by administration of R406, a spleen tyrosine kinase (SYK) inhibitor and the active metabolite of the drug fostamatinib. We showed that SYK and phospho-SYK (the activated form) were present in renal biopsies from patients with IgAN and that SYK expression correlated with the severity of glomerulonephritis.
As a result of this and in collaboration with Rigel Pharmaceuticals, we have established a multicentre international randomized controlled trial (RCT) to examine the effects of SYK inhibition in patients with IgAN whose renal biopsy shows adverse prognostic features.
In collaboration with Baxalta we have also pioneered the development of an anti-macrophage migration inhibitory factor (MIF) monoclonal antibody therapy which is now in Phase I clinical trial. In diabetic nephropathy, or kidney disease, we have identified biomarkers and potential therapeutic targets. For example, urinary CCL2 is a prognostic disease marker and we are part of a successful multicentre RCT of the CCR2 inhibitor for the treatment of diabetic nephropathy. We have identified a receptor for connective tissue growth factor (CCN2) in the kidney biopsies from patients with diabetic nephropathy and found that urinary CCN2 is a prognostic marker.
We have shown that correcting vitamin D deficiency reduces proteinuria and urinary TGF-β1 (a fibrotic factor) in patients with diabetic nephropathy. We have also developed new approaches to glucose monitoring in patients with diabetic nephropathy. We have integrated this discovery to develop a RCT with Boehringer Ingelheim of a new oral hypoglycaemic agent (Linagliptin) in diabetic nephropathy and are monitoring patients using our biomarkers.
Imperial BRC funded studies in renal transplantation have enable us to show that patients with a high variability of tacrolimus levels had worse outcomes with an increased risk of rejection, graft loss and death.
The high variability of tacrolimus levels is associated with patient non-adherence to their medication regime. Patients also had poorer outcomes when tacrolimus levels were maintained outside of the therapeutic range, which reflects clinician non-adherence to protocol.
This led to a change in policy whereby clinicians were directed to modify a tacrolimus dose when one level fell out of range. Following experience gained in organ perfusion research, we have also developed a successful clinical programme for assessing the viability of kidney grafts from extended criteria donors. 15 kidneys have been transplanted so far following machine perfusion with excellent clinical outcomes.