PartnershipTherapeutic Genetic ‘switch’ plays role in cancer invasion and drug resistance
A multi-disciplinary collaboration between Imperial College London and the Institute of Cancer Research identified a genetic ‘switch’ in breast cancer cells that boosts the production of a type of internal scaffolding, aiding in cancer cell migration.
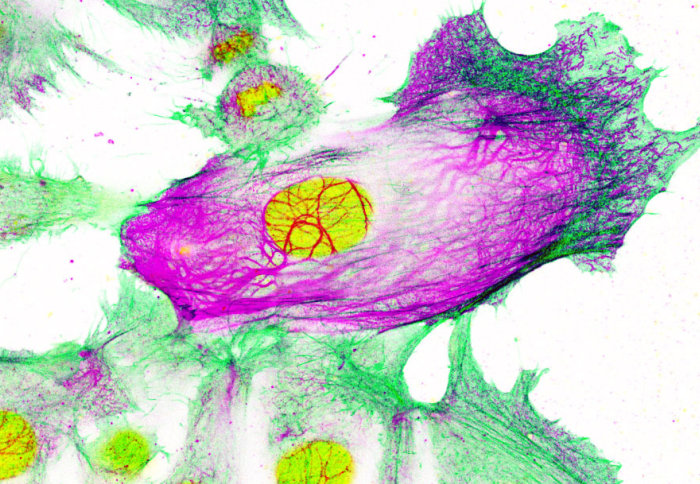
Researchers found that human breast cancer cells resistant to aromatase inhibitor treatment upregulate production of a scaffolding-type protein, called Keratin-80. Build-up of this protein may help cancer cells clump together and travel in the blood stream to other parts of the body. Analysis of patient tumour samples supported these results, demonstrating significantly higher stiffness in cancer lesions than the surrounding normal tissue, particularly at the invasive border. Furthermore, high levels of Keratin-80, measured using immunohistochemistry, positively correlated with tumour stiffness. Finally, high expression levels of Keratin-80 correlated with poor survival in the METABRIC ERα-positive breast cancer dataset, which was especially pronounced in patients who were treated with endocrine therapies and relapsed early.
Dr Luca Magnani, investigator in the NIHR Imperial BRC Cancer Theme, explained further: “Although the spread of cancer around the body affects many patients, scientists are still unsure about the molecular processes that drive the movement of cells. This research sheds light on this process, and also suggests it is controlled by the same switch as drug resistance. These findings need to be replicated in bigger trials, but could potential provide a way of stopping both drug resistance and cancer spread.”
Dr Fernando Calvo, a collaborator from the Institute of Cancer Research and Tumour Microenvironment Team Leader, added: “Our study shows how drug resistance and the invasiveness of cancer cells are interconnected in breast cancer through changes in cell shape. If we understand how to block resistance, we might also be able to prevent the cancer spreading throughout the body – which would be an important step in treating breast cancer more effectively”.
This collaborative study, published in Nature Communications, was supported by Cancer Research UK, with infrastructure support from NIHR Imperial BRC, Imperial Experimental Cancer Medicine Centre and the Cancer Research UK Imperial Centre.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.