Treatment Research emphasises the need for COVID-19 protection for immunosuppressed people
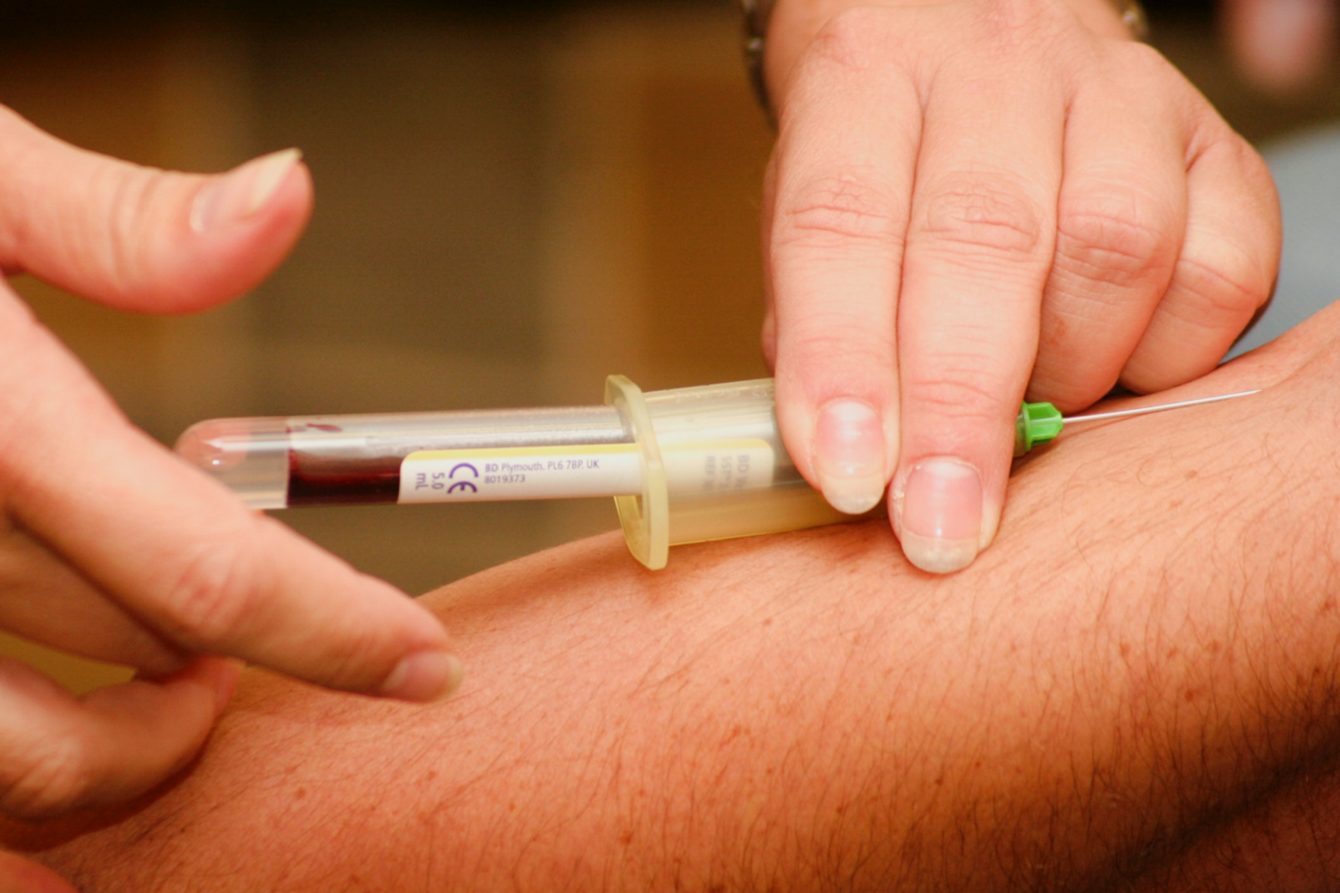
New research, published in The Lancet, reveals how a simple blood test can predict the severity of COVID-19 infection. This could pave the way for personalised approaches to vaccination and better protection for those most at risk.
The MELODY Study explored how well at-risk individuals develop antibodies after at least three COVID-19 vaccinations. Antibodies are crucial for the body to fight off infection, but patients on immunosuppressive medications—common after organ transplants and in the treatment of some types of blood cancer and rare autoimmune diseases—may respond less effectively to vaccines.
Led by Dr Michelle Willicombe from the Department of Immunology and Inflammation, the MELODY (Mass Evaluation of Lateral Flow Immunoassays for the Detection of SARS-CoV-2 Antibody in Immunosuppressed People) Study is the largest of its kind, involving over 28,000 immunocompromised participants—an often-overlooked group in COVID-19 research.
Between December 2021 and June 2022, participants used home-testing kits that involved a simple finger-prick blood test to measure long-lasting antibody levels, employing methods pioneered through the REACT Study one of the world’s largest and most comprehensive coronavirus monitoring studies. Unlike standard COVID-19 tests, these tests checked for immune protection.
The study found that 82% of the patients developed antibodies after vaccination. Those with detectable antibodies were less likely to contract the virus or require hospital care if infected.
Dr Willicombe, Clinical Reader at Imperial College London and Honorary Consultant Nephrologist at Imperial College Healthcare NHS Trust, said: “Although it is really reassuring to see that most patients in this group responded well to vaccination, we know this approach can be less effective in some patients treated with certain immunosuppressive medication, and careful consideration in any protective strategies is required.
“MELODY has shown it is possible to individually assess vaccine response and the risk of severe outcomes following COVID-19 infection in this group. This information has the potential to support personalised approaches to COVID-19 prevention, such as individualised vaccination schedules or treatments, helping at-risk patients to benefit more equally from available public health measures.”
Co-author Professor Graham Cooke, Deputy Dean of the Faculty of Medicine and NIHR Imperial BRC Infection and AMR Theme Co-lead, added: “Many of us used self-reported home testing for the first time during the COVID pandemic. This study, focussed on vulnerable patient groups, is a great demonstration of the potential for self-testing approaches in routine clinical settings as we look to shift NHS care towards prevention in the community.”
The study also revealed broader patterns affecting COVID-19 risks. Patients who continued shielding had higher infection rates once restrictions eased, possibly due to a lack of prior exposure to the virus. Living in a household with children was another factor linked to increased infection rates, highlighting the challenges of preventing household transmission.
Dr Willicombe emphasised: “Vaccination has improved the outcomes of COVID-19 infection considerably for most patients, but we know that we will face outbreaks of viruses in the future and preparing on how to best protect immunosuppressed people is of utmost importance. MELODY has provided valuable information on how best to protect at-risk populations both in terms of behavioural changes, such as shielding, and vaccinating, based on personal circumstances. This knowledge can be applied to inform ongoing COVID-19 risk and also outbreaks of new viruses, should they occur.”
The findings underscore the importance of adapting health measures to meet the needs of vulnerable patients, ensuring everyone benefits from advances in medicine and public health.
Dr Aisling McMahon, Executive Director of Research at Kidney Research UK, praised the study: “This hugely important work has shown that COVID-19 vaccination has protected many kidney transplant recipients from COVID-19. It also demonstrates that we can identify those who remain at risk simply and reliably. We must now build on these findings to ensure that planning for future vaccination strategies is informed by these results and supports the best outcomes for at-risk individuals. I would like to thank all the patients who participated in this groundbreaking study and congratulate everyone involved in MELODY.”
Dr Richard Francis, Deputy Director of Research at Blood Cancer UK, added: “During the pandemic, people with blood cancer who were immunocompromised often faced an uphill battle navigating bureaucracy to get access to repeat vaccines. It caused huge anxiety for this group of people, at a time when they also accounted for one in 20 of COVID-19 intensive care patients. At this time, we didn’t know whether having a weakened immune system also meant that some people wouldn’t generate a ‘good response’ to the COVID-19 vaccines.
“These findings, alongside results from other studies, are very reassuring for many with blood cancer. They tell us that people with weakened immune systems generally respond well to repeat vaccines and that they help prevent hospitalisation. Looking to the future, the research also demonstrates that mass testing of immunocompromised individuals could be possible. Using home tests like this would give people a better idea of individual risk and helps to personalise prevention strategies, whether this be through targeted repeat vaccinations, specific treatment or even management of household contacts.”
The research was supported by Kidney Research UK, UK Research and Innovation (UKRI), Blood Cancer UK, Vasculitis UK, and Cystic Fibrosis Trust. It was conducted in collaboration with the National Disease Registration Service within NHS England by the RECORDER partnership with the University of Nottingham, who carried out all analyses for the Rare autoimmune and Blood cancer cohorts; and in collaboration with NHS Blood and Transplant who carried out all analyses for the transplant cohort, and managed the study through its Clinical Trials Unit.