Therapeutic Facilitating access to organoid research for advanced disease modelling
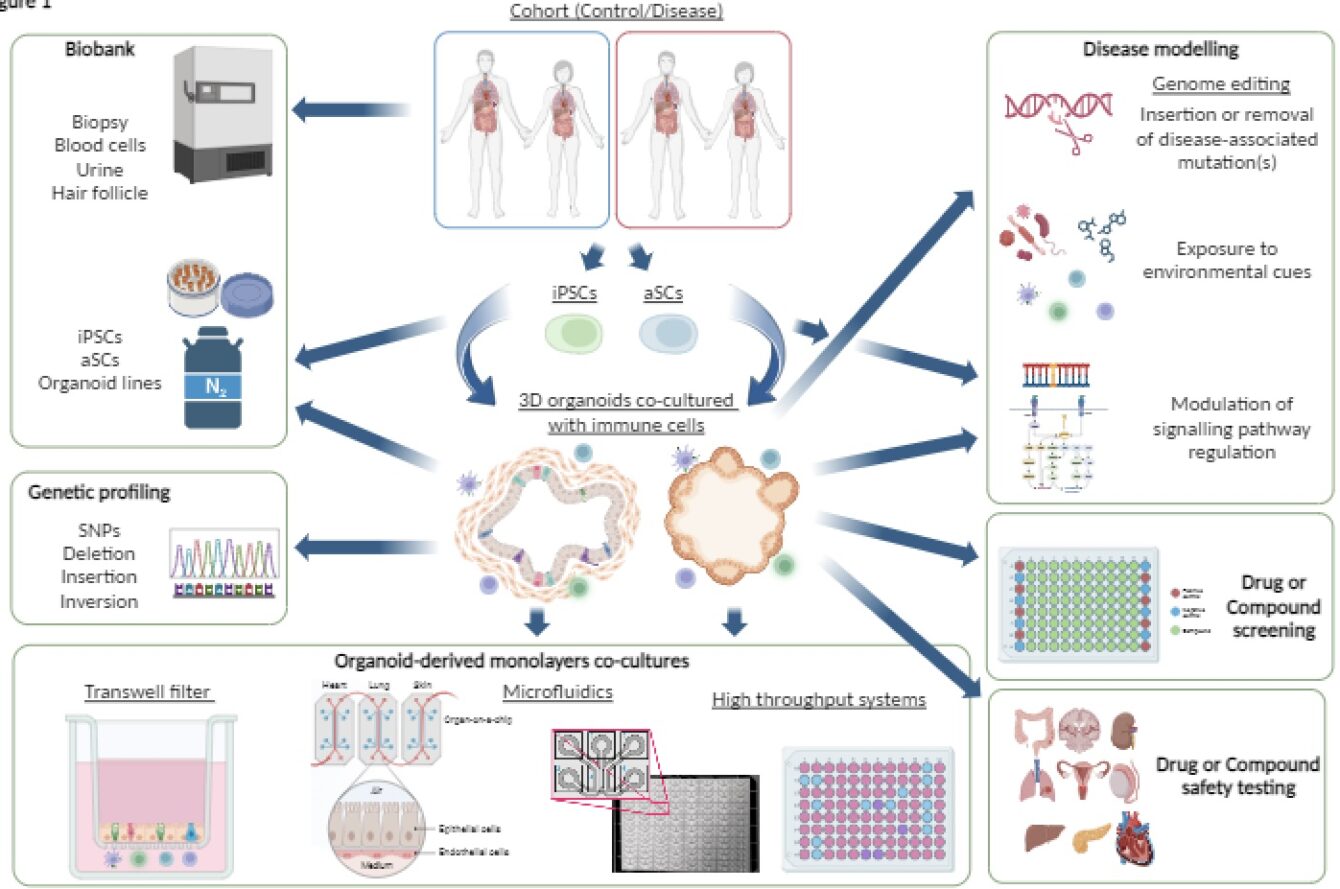
The NIHR Imperial BRC Organoid Facility, established in 2023, supports organoid-related disease modelling and precision medicine efforts for the Imperial College and the Trust by providing organoid lines, coordination, and facilitation of organoid-related activities including training for students and researchers. Dr Tamas Korcsmaros, the facility lead, talks in detail about the facility here.
Through collaboration with Imperial’s Faculty of Engineering and key industrial partners, the Organoid Facility is developing improved methods to overcome challenges surrounding the growing environment for organoids. In this piece, Sandra Koigi, the Laboratory research technician in Organoid Culturing at the Imperial BRC Organoid Facility, talks about the three recently published review papers and how the approaches outlined could support the widespread use of advanced organoid models.
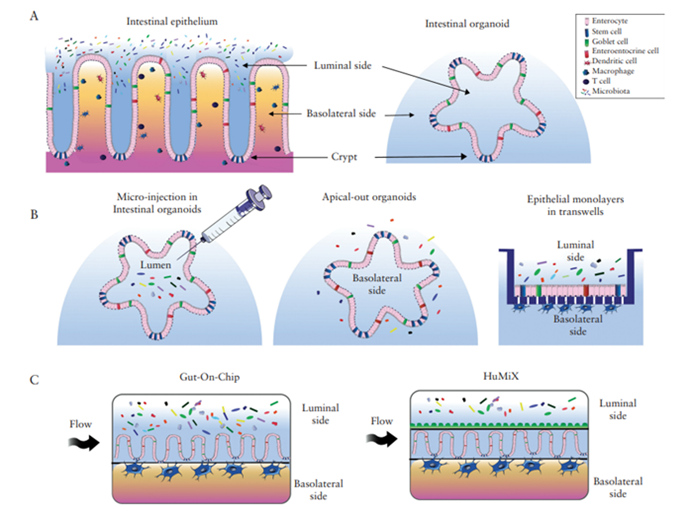
Stem cell technology has made great advancements in the last 20 years. Whilst traditional cell-culture methods are not as efficient in mimicking conditions found in complex diseases, with stem cells, we are now able to create organoids which are 3D structures that can resemble organs. Organoids such as intestinal organoids, can organise into different physiological structures, renew themselves and generally recapitulate the major in vivo functions (Figure 1A).
However, most organoids that are grown in the labs, only contain epithelial cells and lack disease-relevant other cell types, such as immune cells. Thus, co-culturing immune cells with organoids can help researchers to model diseases more accurately. For example, inflammatory bowel disease (IBD) like Crohn’s disease and ulcerative colitis is characterised by an overactive immune response against microbes in our gut. To understand the complex interaction between the host and the microbes, they need to be cultured together along with other cell types such as immune cells.
In celebration of World Organoid Research Day, we present our main approaches and our three recent reviews that we compiled to increase awareness of the different aspects of organoid research at the WORD+ conference this week.
One of our review papers addresses the problem that despite organoids being an extremely powerful tool to use, they can also be challenging for new users. The review by Hautefort et al., 2022 gives a succinct background to stem-cell-based models and the development of 3D organoids. It also highlighted how organoids can be used to study cell-cell and cell-microbe interactions. The paper alerts new users of factors, such as source, types of cells to include, availability of growth conditions and different readouts applicable to specific models when considering which organoid model to use, as well as providing a list of examples of organoid culture approaches.
Organoids are also beneficial for studying different cell types and cell-cell communication. This review highlights a few options for studying this, for example, the use of hydrogel scaffold, co-culture of organoids within hydrogel domes, exposing organoids or monolayers with culture media from different organoids and the use of microfluidics which can control fluid flow into cultures. Also presented, are the challenges that come with studying organoids and the emerging, improved organoid models.
Various methods to study the interaction between the host and microbes in the context of IBD using organoids are outlined in a review by Poletti et al., 2020. One technique involves the microinjection of microorganisms in the inner part of the organoids, where the bacteria typically interact. Alternatively, “apical-out” organoids can be used, where the organoids are manipulated to have their inner part out and researchers can add microbes directly to the culture medium. Another advancement highlighted is converting the 3D organoids into a 2D system by fragmenting the organoids from patient biopsies into small clumps of cells or single cells and plated onto transwell inserts (Figure 1B). The use of microfluidic devices that can mimic the body’s condition more accurately by using chemical gradients, mechanical cues and at times, a controlled gradient of oxygen. These are known as ‘Organoid-on-Chip’ systems including various ‘Gut-on-Chip’ solutions and more advanced systems such as the Human-Microbial Cross-Talk (HuMiX) platform, where anaerobic bacteria can be kept in an almost oxygen-free compartment offer alternative co-culture methods that more closely resemble conditions in the human colon (Figure 1C).
Our most recent review highlights how immune research could benefit from using organoid-based co-cultures and chip systems by Papp, Korcsmaros and Hautefort (2024). This review emphasises the importance of culturing immune cells along with different cell types to more accurately model diseases. For example, tumour-derived organoids co-cultured with immune cells can help researchers understand how immune cells respond to tumours and test potential treatments.
In modelling IBD, co-culture of organoids with immune cells has revealed how certain immune cells affect IBD progression. Tissue regeneration is also studied by culturing organoids with immune cells to uncover how they play a role in stimulating stem cell growth and restoration of tissue function after inflammation. As with co-culture with bacteria, microfluidic systems can also be useful for the co-culture with immune cells. The review of Papp et al provides a detailed list of examples of microfluidic chip systems that can be used for co-culture with immune cells or other cells and provides advantages and limitations of using chips and microfluidics for the culture of organoids and co-culture with other cells.
Overall, we developed this review trio to enable extensive learning about, but not limited to, the development of 3D organoids, how to use them for research, and the importance of co-culturing with immune cells and/ or microbes for better disease modelling.